For example, a crew chief’s responsibilities can contain authorization to use departmental means and interviewing the concerned staff to completely realize the challenge.
QUALIFICATION & VALIDATION.Validation is an essential Element of GMP, and a component of QA.Vital ways in the method need to be validated.Need for self-assurance which the solution will persistently satisfy predetermined requirements and attributes.
A GMP audit is an extensive, 3rd-bash inspection of pharmaceutical creation company or supplier within the pharmaceutical benefit chain.
This shared technique assists pharmaceutical companies manage high-quality requirements although cutting down copy function, delivering Rewards that go beyond preserving dollars.
A skilled GMP auditor who can evaluate and keep track of supply chain stakeholders, can travel higher insight which lets you enhance Manage around good quality. All regulatory agencies who established expectations for your pharmaceutical industry have an expectation of fine Production Follow compliance, such as, across output offer chains.
Generally, just one Shared Audit is made of five to ten buyers’ and in some cases, a Shared Audit will hold as many as 25 consumers’ audit requirements.
If you want to report an Intertek Certified/Tested merchandise that does not seem like compliant, or has long been associated with a mishap, Call us and we will handle your inquiry immediately.
This document discusses auditing of good quality assurance and engineering departments. It defines quality audit and discusses the importance of high-quality maintenance via pillars like concentrated advancement and autonomous routine maintenance.
The solution to this dilemma is, Of course It's really a regulatory necessity. The many regulations obtaining their unique need as per regulatory pointers.
Internal audits Enjoy a essential function in the pharmaceutical industry, supporting companies make certain compliance with regulatory expectations, determine potential pitfalls, and keep the best amount of item high quality and safety. As pharmaceutical companies facial area significantly advanced regulatory requirements and evolving market dynamics, the significance of robust internal audit procedures can not be overstated.
The audit Coordinator shall guide them to some selected convention home or Business for the whole time in the Audit.
This check here document discusses auditing of good quality assurance and engineering departments. It defines excellent audit get more info and discusses the importance of high quality maintenance through pillars such as centered advancement and autonomous maintenance.
The intention of vendor audits is to inspect sellers' quality management units and assure they fulfill necessities for making capsules and sterile medical products and solutions.
It can help to evaluate the efficiency of the Corrective and Preventive steps and improves the remedial actions.
 Molly Ringwald Then & Now!
Molly Ringwald Then & Now!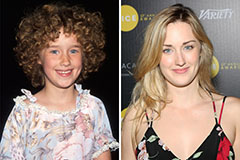 Ashley Johnson Then & Now!
Ashley Johnson Then & Now! Sam Woods Then & Now!
Sam Woods Then & Now! Elin Nordegren Then & Now!
Elin Nordegren Then & Now!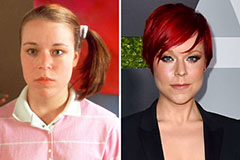 Tina Majorino Then & Now!
Tina Majorino Then & Now!